Articles > Ruthenium Applications
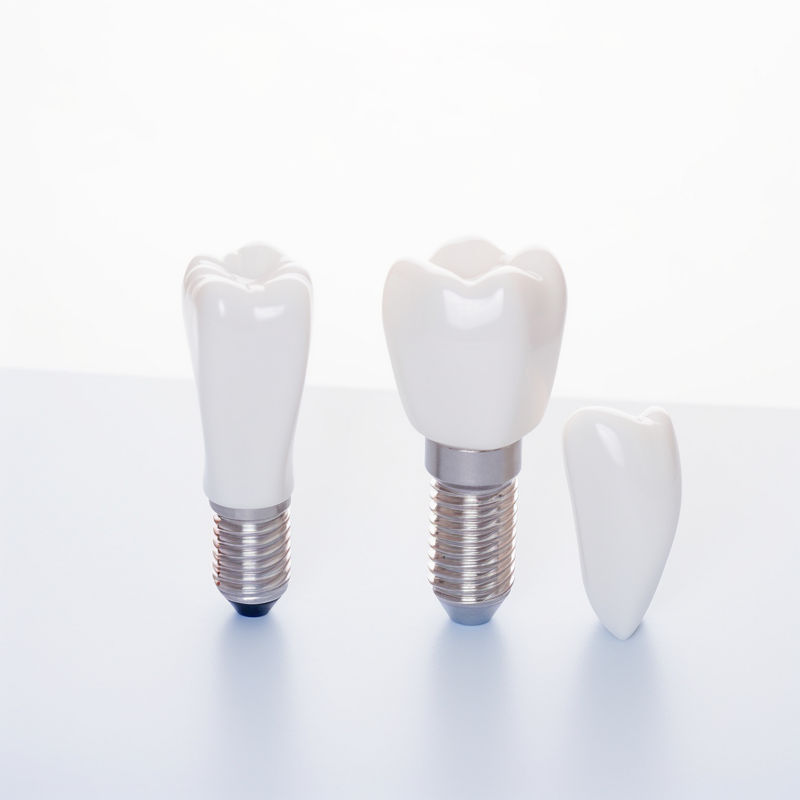
Dental implants are a crucial advancement in dentistry, providing a permanent solution for replacing missing or damaged teeth. There are three main types of dental implants: endosteal, subperiosteal, and transosteal.
Endosteal implants are the most common type and are placed directly into the jawbone. Subperiosteal implants are placed on top of the jawbone but beneath the gum line, and transosteal implants are inserted through the jawbone. Each type has its own unique advantages and is chosen based on the patient's specific needs and jaw structure.
The success rate of dental implants is incredibly high, with over 90% of implants still functioning well after ten years. This highlights their long-term reliability and durability, making them a preferred option for tooth replacement.
In orthopaedic joint replacements, various fixation mechanisms are used to secure joint prostheses. These include cemented, cementless, and hybrid fixation methods, each with its own benefits and considerations. These mechanisms are crucial in ensuring the stability and longevity of joint replacements.
In conclusion, dental implants play a critical role in restoring oral health and function, with a high success rate and long-term reliability. In orthopaedic joint replacements, various fixation mechanisms are used to secure joint prostheses, ensuring stability and longevity.
Ruthenium is a lesser-known but crucial element in the field of dental implants. Its unique properties and characteristics make it an important material in the production and functionality of dental implants. From enhancing the mechanical properties of the implants to improving their biocompatibility and corrosion resistance, ruthenium plays a significant role in increasing the longevity and success of dental implants. In this article, we will explore the various ways in which ruthenium contributes to the effectiveness and reliability of dental implants, and why it is an essential element in the field of dental prosthetics.
Ruthenium is a rare and precious metal that belongs to the platinum group of metals. It is known for its excellent chemical and physical properties, including high corrosion resistance, hardness, and a high melting point. In the context of dental applications, ruthenium is often used in cobalt-chromium dental alloys as an alloying element, particularly in combination with aluminum.
When added to cobalt-chromium dental alloys, ruthenium helps to improve the mechanical properties of the alloy, such as its hardness, strength, and resistance to corrosion. Additionally, the presence of ruthenium in the alloy can also enhance its biocompatibility, making it suitable for use in dental prostheses.
The use of ruthenium in dental alloys is crucial for the manufacturing and performance of dental prostheses, as it contributes to the overall durability and longevity of the prosthetic device. By enhancing the properties of the dental alloy, ruthenium plays a key role in ensuring that dental prostheses can withstand the harsh conditions of the oral environment and provide long-lasting functionality for the patient. Overall, ruthenium is a valuable addition to cobalt-chromium dental alloys, playing a vital role in the production of high-quality dental prostheses.
Noble metals play a crucial role in dental implants due to their exceptional biocompatibility, longevity, and cost-effectiveness. When used in dental restoration, noble metals such as palladium, platinum, and gold offer superior performance and suitability. Their biocompatibility ensures that they are well-tolerated by the body, reducing the risk of allergic reactions, inflammation, or rejection. This makes them a preferred choice for dental implants, as they promote better healing and integration with the surrounding tissues.
In addition, noble metals are highly resistant to corrosion and wear, leading to longer-lasting dental implants that require minimal maintenance or replacement. This translates to cost-effectiveness for both the patient and the dental practice, as the need for frequent repairs or replacements is reduced.
Furthermore, the use of high noble alloys further emphasizes the biocompatibility and longevity of dental implants. These alloys contain a higher percentage of noble metals, enhancing their performance and durability. Overall, the importance of using noble metals in dental implants cannot be overstated, as they not only ensure the success of the dental restoration but also contribute to the overall well-being of the patient.
Ruthenium is a transition metal in the platinum group with unique physical properties. In this section, we will explore the various characteristics that make ruthenium an intriguing element in terms of its physical properties. From its distinctive silvery-white appearance to its high melting point and density, we will delve into the specific physical attributes that define ruthenium. Additionally, we will touch on its unusual ability to exist in several oxidation states, its resistance to corrosion, and its role in the production of alloys and catalysts. Understanding the physical properties of ruthenium is essential for its applications in various fields, including the manufacturing of electronic components, jewelry, and industrial processes.
Gold-based alloys used in dental applications typically have a melting point ranging from 960°C to 1060°C, a density of 12-16 g/cm3, and an atomic weight of around 196.97 g/mol. Varying the composition of these alloys can significantly affect their properties. For example, the addition of palladium, platinum, or silver can increase the melting point and mechanical strength of the alloy, while the addition of copper, zinc, or other platinum group metals can also affect these properties.
In dental gold alloys, a minimum gold content of 75% is required to ensure biocompatibility and corrosion resistance. Non-precious metallic elements like nickel, cobalt, or chromium are often added to gold alloys for use in metal-ceramic reconstructions, to enhance the mechanical properties and minimize the risk of corrosion.
Overall, the composition of gold-based alloys in dental applications plays a crucial role in determining their physical and mechanical properties, ensuring their suitability for use in various dental restorations.
Dental alloys and implants have a crystal structure that is formed through the bonding of metal atoms. This structure consists of individual grains, with grain boundaries separating the grains. Metallic bonding holds the atoms together within each grain, giving the material its strength and durability.
Gold alloys used in dental bonding undergo a heat-treating process to strengthen the material and improve its bonding to tooth tissue. This process involves heating the alloy to a specific temperature and then cooling it at a controlled rate to create the desired properties.
In terms of disinfection, metal and metal-ceramic restorations require a specific regime to ensure proper cleaning and maintenance to prevent infection and deterioration.
Common materials used for dental implants include SS316L, cobalt chrome alloy, and titanium. Each material has distinct characteristics such as strength, corrosion resistance, and biocompatibility, making them suitable for different implant applications. The wear mechanisms of these metallic dental implants vary depending on the material used and the specific conditions they are subjected to in the oral environment.
Ruthenium is a rare transition metal with unique mechanical properties that make it an important material in various industrial applications. In this article, we will explore the mechanical properties of ruthenium, including its strength, hardness, ductility, and toughness. Understanding these properties is crucial for designing and manufacturing components for the aerospace, electronics, and chemical industries. We will also discuss the ways in which ruthenium's mechanical properties can be enhanced through alloying and heat treatment, as well as its potential for use in advanced materials and technologies. Whether you are a materials scientist, engineer, or simply curious about the fascinating world of metals, this article will provide valuable insights into the mechanical behavior of ruthenium and its role in modern technology.
Tensile strength is the ability of a material to resist breaking under tension. Ductility refers to a material's ability to deform without breaking when subjected to tensile stress. Hardness, on the other hand, measures a material's resistance to surface indentation or scratching.
These material properties are important for understanding how a material will behave when subjected to mechanical forces. A material with high tensile strength will be able to withstand pulling or stretching forces without breaking, while a highly ductile material can be stretched or bent without fracturing. A hard material will resist surface indentation or scratching, making it suitable for applications where wear and tear is a concern.
Mechanical testing is used to measure these properties, allowing engineers and designers to select the most appropriate material for specific applications. For example, a steel cable needs to have high tensile strength to bear heavy loads, while a metal used in forming processes needs to be ductile to be shaped without breaking. Similarly, tools or components that are subject to wear and tear should have high hardness to resist surface damage.
Zirconia is a ceramic material frequently used for dental implants. It is biocompatible and exhibits high corrosion resistance, making it a suitable option for dental applications. Zirconia is also aesthetically pleasing, as it closely resembles natural teeth. However, it is more brittle compared to titanium-based alloys, which may be a concern for long-term durability.
Polymeric materials, such as polymethylmethacrylate (PMMA) and polyetheretherketone (PEEK), are also used for dental implants due to their biocompatibility and low weight. However, they may not have the same mechanical strength as titanium-based alloys, making them more prone to wear and tear over time.
In comparison to titanium-based alloys, zirconia and polymeric materials may have superior biocompatibility and corrosion resistance. However, they generally have lower mechanical strength, which may compromise their long-term effectiveness as dental implants. Therefore, the choice of implant material should be carefully considered based on the specific needs and characteristics of the patient.
Ruthenium is a transition metal known for its various chemical properties that make it valuable in a wide range of applications. From its ability to resist corrosion to its catalytic properties, ruthenium displays a diverse array of chemical behaviors that make it a vital component in many industrial processes and everyday products. Understanding the chemical properties of ruthenium can provide insight into its usefulness in fields such as electronics, jewelry, and energy production. This article will explore the key chemical properties of ruthenium, including its reactivity, oxidation states, and coordination chemistry, shedding light on the unique characteristics that make this element so valuable in the world of chemistry and materials science.
Surgical alloys, such as stainless steel and titanium, generally exhibit low reactivity with acids and bases due to their chemical composition, which often includes a high proportion of non-reactive metals like iron and chromium. Polymers, on the other hand, can have varying reactivity with acids and bases depending on their specific chemical makeup. Some polymers, like polyethylene and polypropylene, are relatively inert and resistant to chemical reactions, while others, such as PVC, can be more reactive.
Saliva, with its natural composition of water, electrolytes, mucus, and enzymes, can also exhibit reactivity with certain chemicals. For example, saliva contains bicarbonate ions, which act as a buffer and help maintain a neutral pH, providing some resistance to acidic or basic substances.
Specific standards or specifications for the reactivity of these materials are often outlined by regulatory agencies, such as the FDA or ASTM, to ensure that surgical alloys and polymers meet certain safety and performance criteria. These standards can include requirements for corrosion resistance, chemical compatibility, and biocompatibility. Understanding the chemical composition of these materials is crucial in predicting and controlling their reactivity with various substances.
Corrosion resistance in the oral environment is influenced by several factors. The pH of the oral environment, which can fluctuate due to consumption of acidic foods and drinks, can affect the corrosion resistance of dental materials. Additionally, the presence of electrolytes, such as saliva, and oxygen compositions can also contribute to corrosion. The buildup of dental plaque can further exacerbate corrosion by creating localized areas of low pH and high concentrations of corrosive substances.
Galvanic corrosion, stress corrosion cracking, and fretting corrosion are all potential concerns for dental implants. Galvanic corrosion can occur when dissimilar metals are in contact, leading to accelerated degradation. Stress corrosion cracking can occur under tensile stress, which can weaken the implant. Fretting corrosion can occur due to mechanical wear and tear in the oral environment, leading to localized corrosion.
Metallic materials, such as stainless steel and titanium, are commonly used in dental implants and generally exhibit good corrosion resistance. Ceramic materials, such as zirconia, also show excellent corrosion resistance due to their inert nature. Polymer materials, while generally less resistant to corrosion than metals and ceramics, can still be used effectively in the oral environment with proper formulation and care. Understanding the factors that contribute to corrosion resistance and the specific challenges faced in the oral environment is crucial for the long-term success of dental materials.
Ruthenium Alloys for Dental Implants:
Ruthenium, a rare and precious metal, has found its way into the field of dentistry due to its exceptional properties that make it an ideal material for dental implants. Ruthenium alloys offer superior strength, corrosion resistance, and biocompatibility, making them an excellent choice for long-term dental solutions. With its ability to withstand the harsh oral environment and provide stable support for prosthetic teeth, ruthenium alloys have become increasingly popular in the development of dental implants. In this article, we will explore the unique characteristics of ruthenium alloys and their applications in the field of dentistry, as well as their potential benefits for patients in need of dental restoration.
Incorporating ruthenium into titanium alloys can significantly enhance their properties, offering a range of advantages. First, ruthenium can effectively reduce the stiffness of titanium alloys, making them more suitable for use in medical implants. This reduced stiffness allows for better mechanical similarity to bone, which can promote better integration and reduce the risk of bone stress shielding.
Furthermore, the addition of ruthenium can greatly improve the corrosion resistance of titanium alloys. This is particularly crucial in medical applications, as it ensures the long-term durability and reliability of implants within the body. Ruthenium also enhances the biocompatibility of titanium alloys, reducing the risk of adverse reactions and promoting better tissue response.
Overall, the incorporation of ruthenium into titanium alloys offers a range of advantages, including improved mechanical similarity to bone, reduced stiffness, enhanced corrosion resistance, and better biocompatibility. These benefits make ruthenium-enhanced titanium alloys a valuable option for various medical and orthopedic applications.